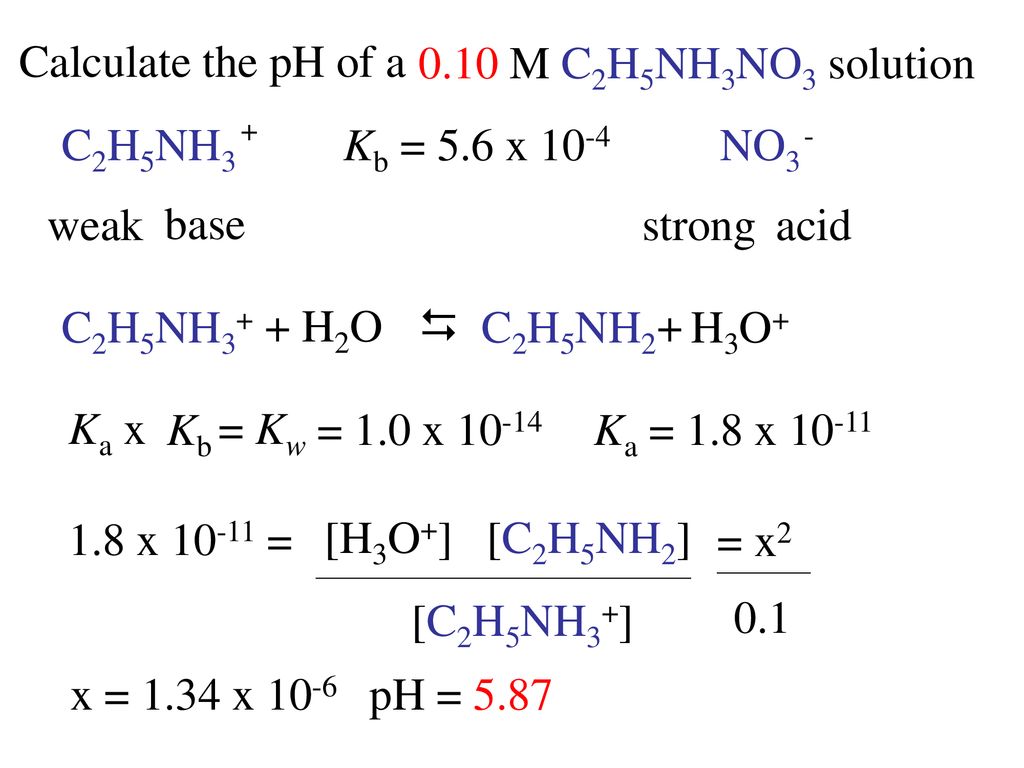
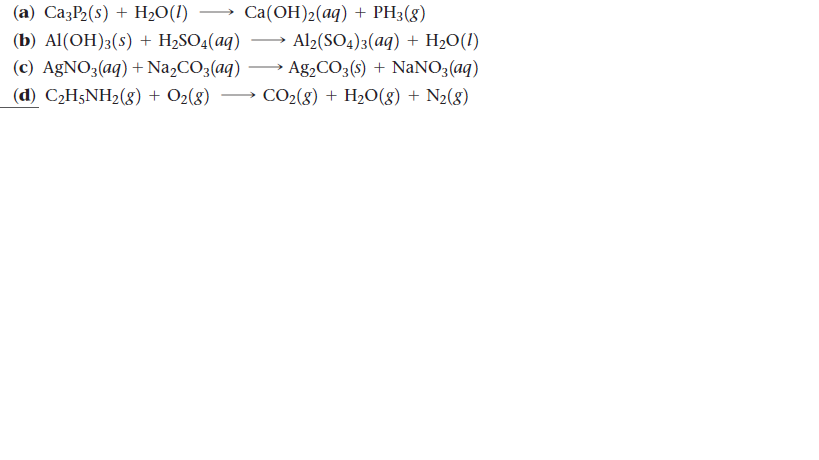Max. Marks : 70 an 4. Complete the following reaction. CO → A -KI NH KOH (alc.) CHI NH H2O CO - HO C + C2H5NH2 Ethylamine
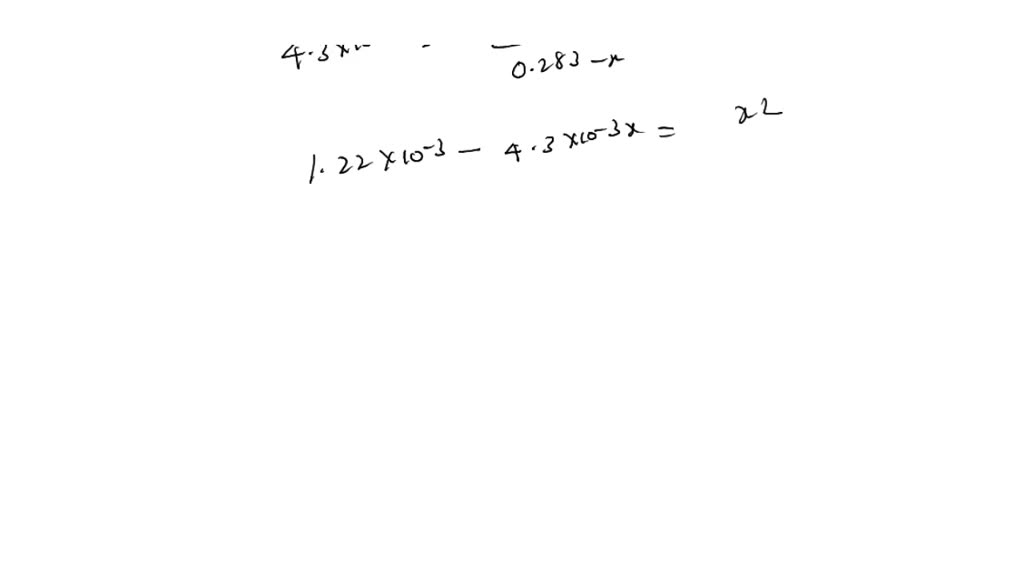
SOLVED: C2H5NH2 + H2O <–> C2H5NH3 +OH- A 0.283 M solution of C2H3NH2 was created. Calculate the equilibrium concentrations of all species, and the pH of the solution.
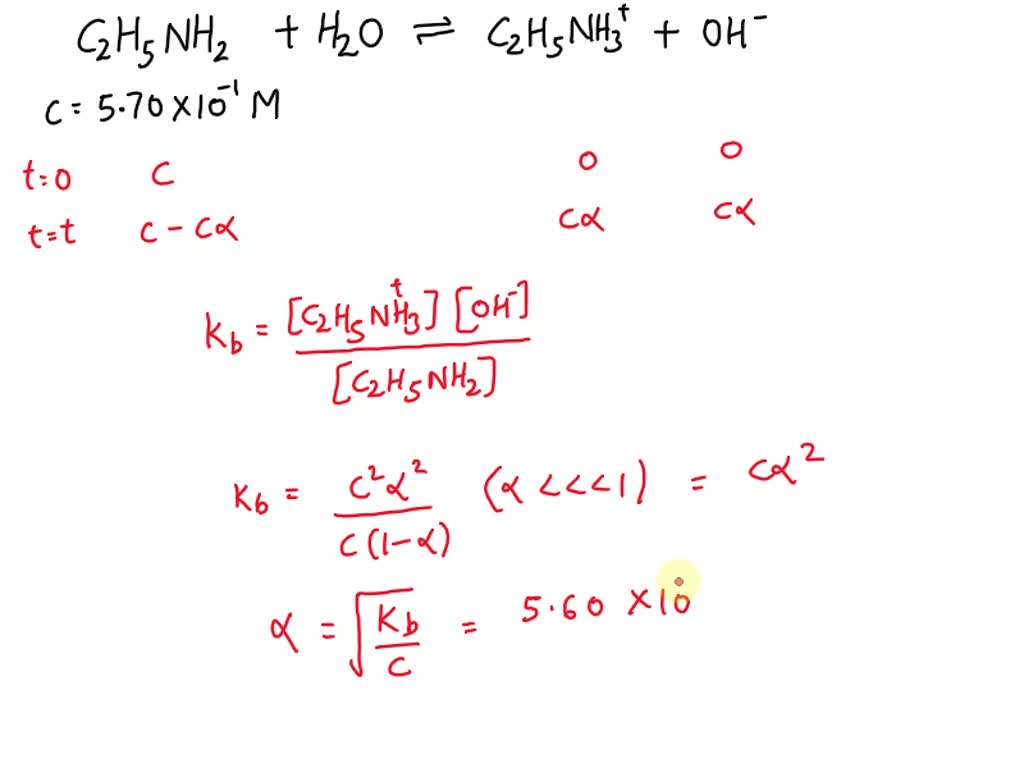
SOLVED: Calculate the pH of a 5.70×10^-1 M aqueous solution of ethylamine hydrochloride (C2H5NH3Cl). (For ethylamine, C2H5NH2, Kb = 5.60×10^-4.)
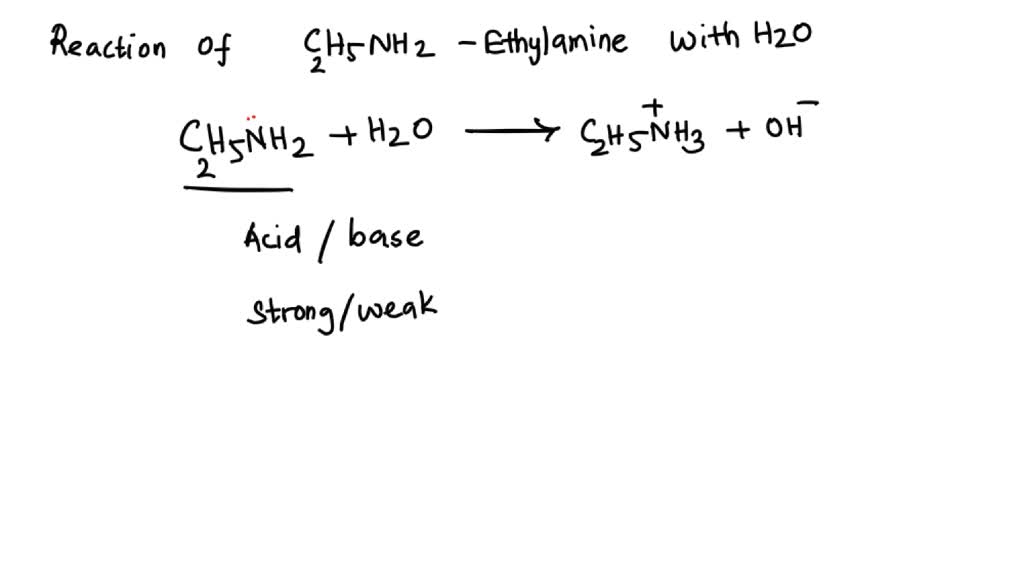
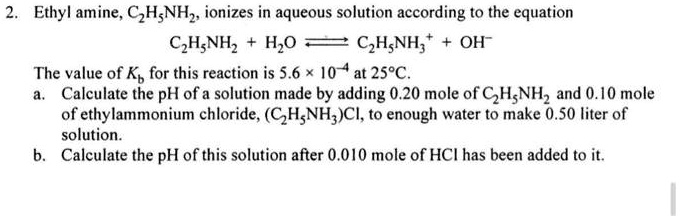
SOLVED: 5. When added to water, ethylamine undergoes the following reaction: C2H5NH2 + H2O C2H5NH3+ + OH- Is ethylamine an acid or a base in this reaction? Strong or weak? How do
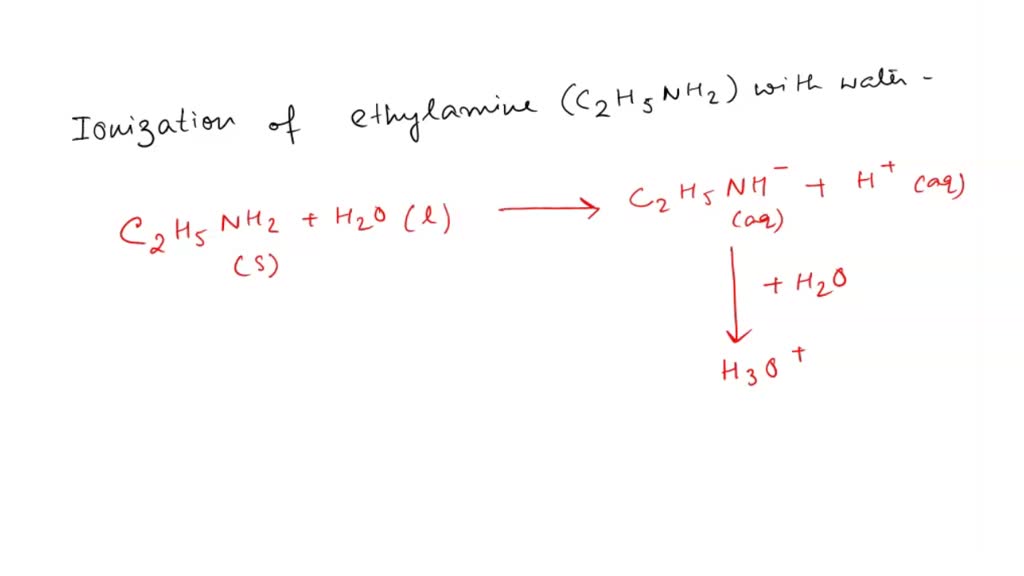
SOLVED: Write the equation for the ionization of ethylamine (C2H5NH2), a weak molecular base, with water
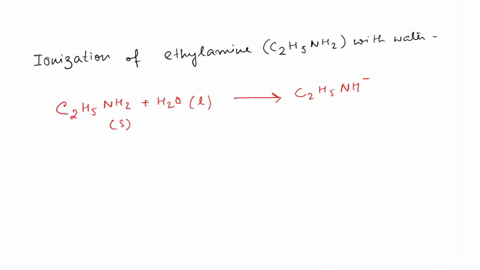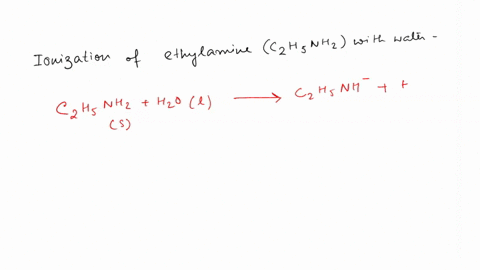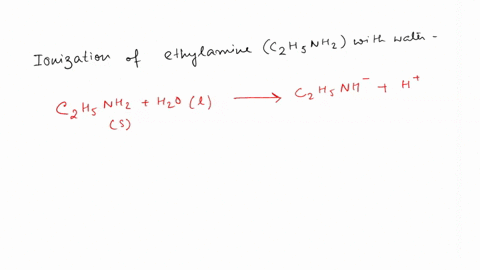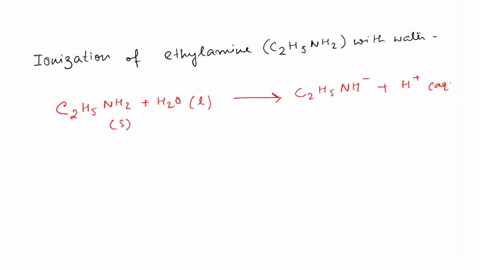
SOLVED: Write the equation for the ionization of ethylamine (C2H5NH2), a weak molecular base, with water
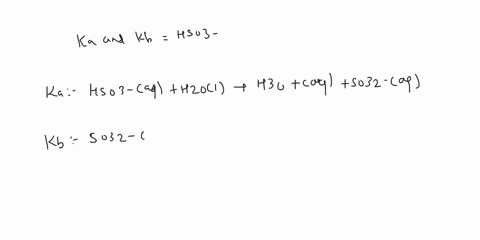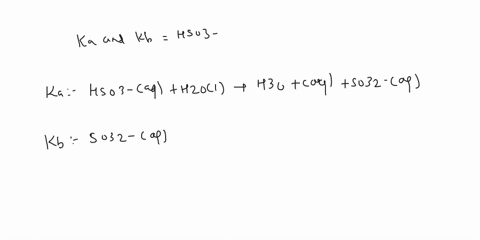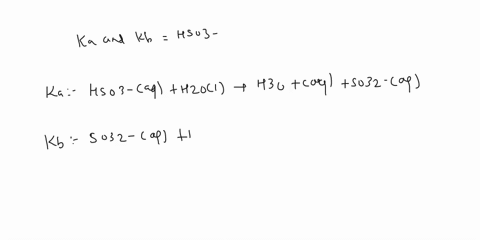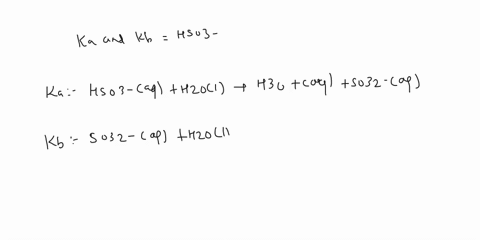
SOLVED: a.) CO3^2- + H2O -> HCO3^- + OH- b.) C6H5NH2 + H2O -> C6H5NH3^+ + OH- c.) C2H5NH2 + H2O -> C2H5NH3^+ + OH-