![PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e2ac2e88133be5a7278abdd433a3dd75d426a246/2-Figure1-1.png)
PDF] Competition between Al2O3 atomic layer etching and AlF3 atomic layer deposition using sequential exposures of trimethylaluminum and hydrogen fluoride. | Semantic Scholar

Inhibition of AlF3·3H2O Impurity Formation in Ti3C2Tx MXene Synthesis under a Unique CoFx/HCl Etching Environment | ACS Applied Energy Materials
![The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1293255816303363-fx1.jpg)
The missing hydrate AlF3·6H2O [Al(H2O)6]F3: Ionothermal synthesis, crystal structure and characterization of aluminum fluoride hexahydrate - ScienceDirect

The acid strength of the datively bound complexes involving AlF3 lone pair acceptor and various lone pair donors - ScienceDirect

The molecular graphs of the AlF3-C2H2 (the top left), AlBr3-C2H4 (the... | Download Scientific Diagram
![PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6ecc39c373f3ffa84ca5f44bf21fda67029c3564/4-Figure1-1.png)
PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar

The isomeric structures of the HClO4/n(AlF3) superacids (for n = 1–2).... | Download Scientific Diagram
Inhibition of AlF3·3H2O Impurity Formation in Ti3C2Tx MXene Synthesis under a Unique CoFx/HCl Etching Environment

Possible Formation of H3O+ Cations Due to Aluminum Fluoride Interactions with Water. | Semantic Scholar
![PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6ecc39c373f3ffa84ca5f44bf21fda67029c3564/8-Figure4-1.png)
PDF] STABILITY RELATIONS OF ALUMINUM HYDROXY-FLUORIDE HYDRATE, A RALSTONITE-LIKE MINERAL, IN THE SYSTEM AlF3–Al2O3–H2O–HF | Semantic Scholar

Possible Formation of H3O+ Cations Due to Aluminum Fluoride Interactions with Water. | Semantic Scholar

PDF) Stability of the AlF3 surface in H2O and HF environments: An investigation using hybrid density functional theory and atomistic thermodynamics | Sven Schroeder - Academia.edu
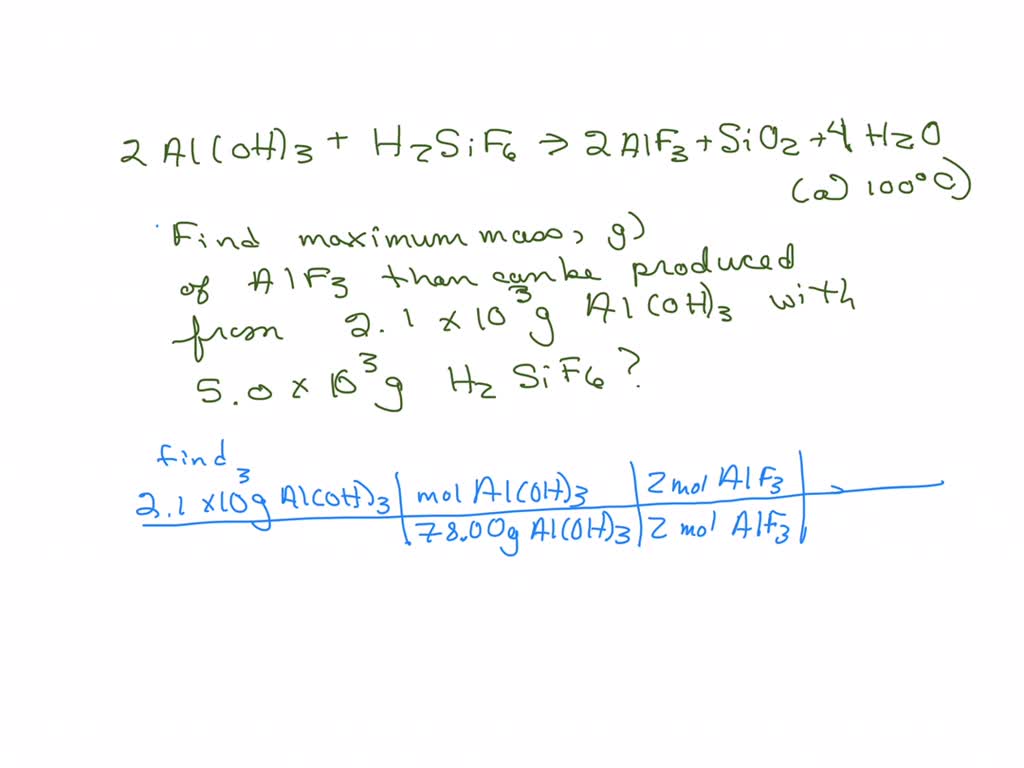
SOLVED: The equation for one process for making aluminum fluoride follows. What is the maximum mass, in grams, of aluminum fluoride, AlF3, that can be produced from the complete reaction of 2.1

Hydrate de fluorure d'aluminium, Puratronic , 99,99 % (base métallique), Thermo Scientific Chemicals | Fisher Scientific